About Us
JOS Pharmaceuticals, a clinical stage pharmaceutical company, has pioneered the se•d8™ wafer, an orally dissolving, synthetic CBD drug. Se•d8™ is for use as a prescription premedication for specific medical procedures that require an awake, conscious sedation anesthetic technique.
Purpose
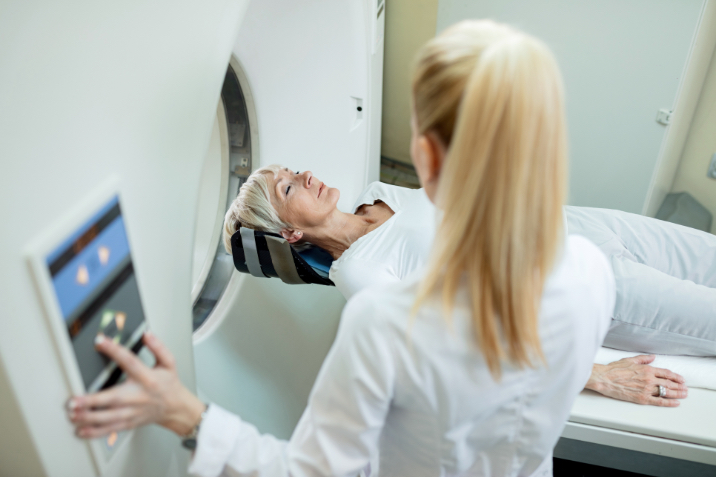
Cataract surgery, LASIK and MRI imaging (performed within the tunnel enclosure machine) require a relaxed, comfortable yet awake and cooperative patient. Through research and development, the well-documented analgesic and sedative effects of cannabidiol (CBD) have been used to create a premedication to benefit both patient and physician.
The se•d8™ wafer drug delivery system is designed to allow direct absorption into the systemic circulation, bypassing both gastric and first-pass hepatic breakdown thereby enabling a more rapid and reproducible clinical response.
New FDA Drug Approval
Our proprietary, entirely synthetic CBD, super-lingual, drug delivery system is currently entering the NDA submission 505(b)(2) Phase 2 pathway for safety, efficacy and dose ranging clinical trials.
Delta-9 is 0%
Ours is an entirely synthetic cannabidiol without THC.
FDA
NDA Submission
Our proprietary, all-synthetic CBD, super-lingual, drug delivery system is currently entering the FDA New Drug Approval (NDA) submission 505(b)(2) Phase 2 pathway for safety, efficacy and dose ranging clinical trials.